Tag Archive for: food safety
Food safety alert update (July 2022) by Safefood 360° RISK assessment tool
in blog, Data report, Food Safety Industry NewsFood safety alert update (January – May 2022) by Safefood 360°
in Data report, blog
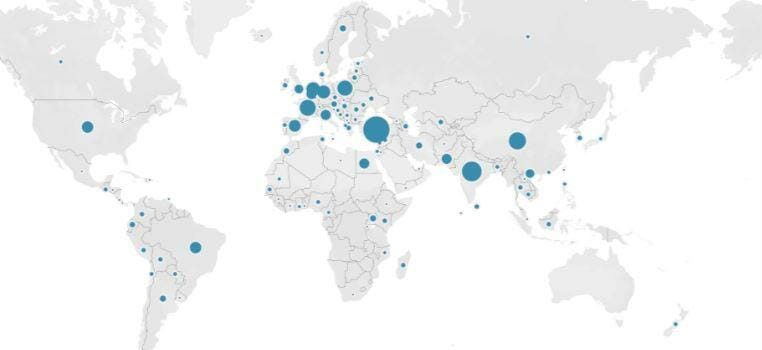
According to our Safefood 360 ° RISK assessment tool report, there are over 1,723 food safety alerts recorded in worldwide.
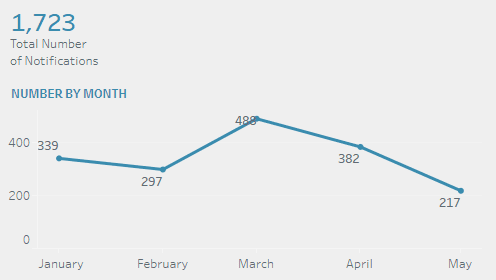
January: 339 cases
February: 297 cases
March: 488 cases
April: 382 cases
May: 217 cases
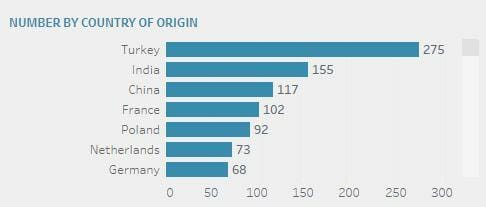
The top 5 alerts come from (in descending order): Turkey (273), India (150), France (95) and Poland (89).
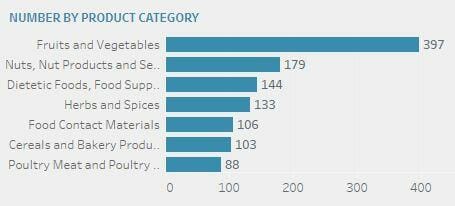
The top 5 alerts are categorised (in descending order): Fruits and vegetables (397), nuts & nuts related product (179), dietetic foods & food supplement (144), herbs & spices (133), and food contact materials (106).
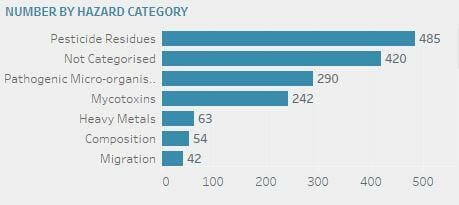
The top 5 alerts relate to (in descending order): Pesticide residues (485), not categorised (420), pathogenic micro-organisms (290), mycotoxins (242), and heavy metals (63).
(To understand the hazards, download our technical datasheet)
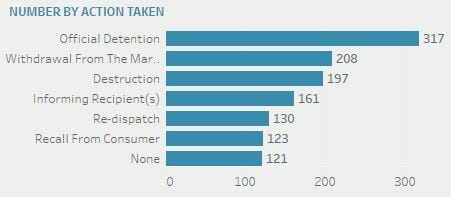
The top 5 actions taken are (in descending order): Official detention (317), withdrawal from the market (208), destruction (197), informing recipients (161), and re-dispatch (130).
On average, there are at least 5-10 food safety hazard alerts in the world everyday that can directly affect your food business and supply chain.
Click here to talk with our technical specialists about how we can help.
What is the ‘New Normal’ for Food Safety?
in blog, Food Safety KnowledgeIn 2001, I remember thinking that life in the United States would never again be the same as it was prior to September of that year.
Sure enough with the turn of time, the term ‘food defense’ quickly entered the public vernacular as new standards began to materialize governing food security and vulnerability was common as a popular sentiment that supposed that food supply chains could be the next target.
Now almost 20 years later, it’s safe to say the world might never be the same again with the Coronavirus impacting every nation globally and posing a ‘9/11 a day’ to those of us in the US.
Thomas Fuller was one of the first in the English language to make a living from his writings, and to anyone that knows an aspiring writer, rather aptly once remarked that “all things are difficult before they are easy”.
All things are difficult before they are easy
Despite being coined nearly a half-millennium ago, I believe this sentiment is still as true as the day the ink used to pen it was wet.
It seems to me the food industry sits on the precipice of unbridled changes which arguably have already begun in earnest as an immediate response to the challenges proposed in 2020 and are likely to reverberate for many more years to come.
What has changed?
At the micro-level, shutdowns forced the temporary closure of businesses across industries stunting the global economy.
While on a larger level, the DOW, NASDAQ, FTSE, or whatever other metrics you might use to evaluate economic health are well-exceeding expectations in spite of what those on the forefront of commerce are experiencing, with many anecdotal stories of austerity emerging.
As the world begins to re-emerge from their shelters, those involved in the food supply chain have been working tirelessly to bring it back to its former strength in spite of significant strain.
It is probable that business is not going to return to the way things were prior to March 2020
To be clear, I truly believe it is probable that business is not going return to the way things were prior to March 2020 ever again.
This was a suspicion I held personally that was all but reconfirmed to me when the FDA announced their Blueprint for the New Era of Smarter Food Safety which was quickly adapted to the learnings from Covid-19 and which intend to provide enhanced tools for the new challenges we have.
For the FDA to react so quickly, shows they acknowledge the significance of the current climate
As I discussed in a previous blog on FSMA which was the last time the FDA made such significant change, monoliths often move slowly and cautiously out of a desire for permanence, so for them to react so quickly and so prominently, shows that they have acknowledged the significance of the current climate and embraced what Thomas Fuller would call the “hard” part in order to make things easier for the industry into the well far-flung future.
What can be done differently?
Prior to the COVID19 pandemic, food safety professionals could ‘get by’ having their food safety and supplier quality management systems simply documented on paper or tucked away on an excel spreadsheet as long as these programs were accessible by their staff who were often within arm’s reach of each other, typically working in the same office.
Prior to COVID-19, food safety professionals could get with legacy systems as long as they were accessible
The merits of the sustainability and efficacy of such an approach was not without its detractors (including myself) but ultimately such systems were endemic in the industry by virtue of their legacy and the fact there was a low barrier of entry to start with them.
In these, HACCP programs, along with their complimentary SOP’s, were able to be tucked away in rudimentary document control systems and oversight was typically limited to direct observations performed by the QA Manager whenever they had an opportunity to walk the production lines, or whenever they were called to respond to a crisis out on the floor.
Audits were typically limited to predefined checklists of food safety standards and rigid in their nature, and when they would need to be changed and documents versioned, unless you had the resources it was a can of worms that Pandora herself might delay opening.
Audits were typically limited… and unless you had resources, it was a can of worms that Pandora herself might delay opening
As a food safety issue occurred, communication of it could be cumbersome as it might require copying and scanning records, then emailing them to relevant individuals where it might sit last in their ‘inbox’ and go unseen causing unnecessary delays.
When an audit involved an external party, such as those from a Certification Body conducting one on behalf of the SQF or BRCGS, they would need to be hosted on-site, often for a day or two, and be given free reign over the environment, something that is just no longer feasible.
The Coronavirus brought about a paradigm shift in our society with things like social distancing, wearing masks, and excessive hand washing.
One of the most obvious and long last impacts of the Coronavirus to the food industry that is quickly becoming apparent is an acceleration of the digital thread of transformation and a wake-up call that just because we can do things one way, doesn’t mean we should continue to do so when there are safer and more pragmatic options available, even if it does mean an element of strain to configure these new approaches in the immediate term.
Just because we can do things one way, doesn’t mean we should continue to do so
In the food industry, we’ve seen additional changes recommended by the CDC such as daily health checks, workplace hazard assessments, and improved building ventilation systems.
Workforce management became more prominent than ever with the CDC not just recommending best practices for contract tracing and proximity tracking, but a myriad of activities and initiatives that businesses could deploy to assist in the fight against Covid-19.
The food industry, in particular, was, and still is, under a tremendous strain with many different challenges arising across sectors from manufacturers to restaurants with many implementing split shifts, reduced workforces, and by consequence, risk running a more ‘light-touch’ approach to compliance than they should ultimately strive for.
What should be done differently?
The world itself has changed, and the food industry needs to adapt along with it.
It is widely accepted that necessity is the mother of all invention, however, in this instance, there is no need to reinvent the wheel, but it might be better to acknowledge that necessity is the mother of adaptation, not invention.
While the principles of food safety systems may essentially remain the same, the old models might not.
While the principles of food safety systems may essentially remain the same, the old models might not
The acknowledgment from GFSI and its related schemes to allow the use of ICT and hybrid audits shows that industry is willing to accelerate the ‘Digital Transformation’ to ‘Industry 4.0’.
As a result, what this means in practical terms is that Food Safety Managers will now be more reliant on technology to perform tasks like communication, remote auditing, and medical screening.
At its core producing and providing safe quality food to consumers is something that is a requirement that remains unchanged, but how we meet these requirements must adapt to circumstance.
Providing safe quality food remains unchanged, but how we meet these requirements must adapt to circumstance
It is all but inevitable that we are likely to see many more changes in the coming months and years, and technology will play a large role in these changes.
Safefood 360° provides the platform from which your company will remain agile enough to maintain accountability in remote working environments while retaining the flexibility to make changes and scale as your business grows.
Remote food safety management is now a real and viable possibility with an agile platform that allows users to log in from virtually anywhere and communicate in real-time, meaning not only can internal audits be performed against any of the 100’s of integrated food safety checklists (GFSI, FDA, Tesco, etc.), but interim guidance checklists which adheres to the World Health Organization (WHO) and Food and Agriculture Organization of the United Nations (FAO) can also be used daily to confirm best efforts are being applied.
Audits both from retailers and technical standards, as well as from the legislators, reflect that manufacturers are acting in the best interests of our consumers, and technology is an enabler that allows us to meet these standards in as efficient a manner as possible.
To hew out of the mountain of despair a stone of hope
Another quote I am fond of is Martin Luther King Jr’s is that with the right mindset “we will be able to hew out of the mountain of despair a stone of hope”.
Some people also believe that nothing worth having comes easy, but to meet these challenges for now and the future, it seems that recent changes have laid the foundational principles to accelerate our industry into the future.
Changes have laid the foundational principles to accelerate our industry into the future
I truly believe that looking forward, Safefood 360° offers the tools for us to lay these stones and build a better industry for us all well into the future.
I am excited for the years ahead and look forward to speaking with you all about creating a long-lasting legacy and positive changes for our industry.
Understanding the FDA “New Era for Smarter Food Safety Blueprint”
in blog, Food Safety KnowledgeBy now you have likely heard that the FDA is introducing some new changes that will affect how businesses in the United States will perceive, process, and demonstrate food safety compliance.
I know what you may be thinking, that this all sounds familiar and have we even finished the most recent overhaul?
It’s important to be clear on what these new changes mean and we can start by understanding that these are not designed to be a replacement of FSMA, but rather a continuation, or to use the FDA’s own terms, the ‘next step’ after FSMA.
Essentially, FSMA prepared the country’s food industry by providing much needed (and long overdue) guidance to businesses in the first major piece of federal legislation for food safety in more than 70 years.
These rules equipped businesses with the necessary tools to build scientific and risk-based protections within the food safety programs, as well as helping companies develop their supplier programs to ensure that hazards would be addressed sufficiently and that adequate controls were put in place when and where identified.
Now, the FDA has recognized that even a powerful piece of legislation like FSMA alone is not enough, and that modern problems require modern solutions.
The FDA has recognized that a powerful piece of legislation like FSMA alone is not enough, and that modern problems require modern solutions
Since FSMA was signed into law in 2011, technological solutions have advanced at a significant rate and can now be utilized to multiple means that were not possible just 10 years ago.
Not only that, but attitudes to food safety have changed with an ever-growing increase on food safety culture being required by standards such as the BRCGS.
With this in mind, it shows the FDA accepts that FSMA was only the first piece needed to reduce foodborne illnesses over the long term.
Enter: the New Era for Smarter Food Safety Blueprint.
In other words, FSMA was the foundation. The New Era is the rest of the house!
FSMA was the foundation. The New Era is the rest of the house
What is in the blueprint for the new Era?
Although the timing of the release seems coincidental with the latest challenges facing the global food industry in respect of COVID-19, these goals have been in motion from some time from the FDA, and are arguably arriving at a most crucial hour when they may be needed most.
Make no mistake, the FDA is not simply throwing this against the wall to see what might stick.
Starting in 2019, over 100 industry experts met to brainstorm what would become the 4 core elements of the New Era:
-
- Tech-Enabled Traceability
- Smarter Tools and Approaches for Prevention and Outbreak Response
- New Business Models and Retail Modernization
- Food Safety Culture
In order to understand what these tenets may mean it is important to understand the reason for each in isolation.
Below is an examination of each ‘Core Element’ and their future potential ramifications.
Core Element 1: Tech-Enabled Traceability
Even with all of the modern advancements in technology, a large portion of the food industry still relies on a paper-based system to manage its supply chain program.
The ability to easily trace products back to their source is cumbersome, to say the least, and during an outbreak when time is critical, a reliable and more agile system is of utmost importance.
When the veracity of data cannot be trusted or shared in real-time, the risk of erroneous data and information may compromise the ability to conduct an effective recall at worst,or introduce distrust to the system at best.
Admittedly, the FDA’s first step here will be to complete FSMA Section 204 rulemaking in order to harmonize the key data elements and critical tracking events needed for enhanced traceability.
This will lay the foundation for traceability which will allow stakeholders within the supply chain to implement digitally-enabled technologies, enable data sharing, and introduce practices all of which will reduce the time it typically takes to trace potentially contaminated foods that are associated with a recall or foodborne illness.
It is important to note that like all blueprints, this is really a plan that outlines a ‘best case’ scenario of what good looks like for the FDA.
In this Element alone the FDA will have a considerable challenge on their hands as they acknowledge that while industry will be encouraged and incentivized to adopt new technologies, it also needs to harmonize the U.S. with regulatory counterparts.
Achieving this by seeking systems interoperability may prove to be a bridge too far as the FDA could likely face a delicate balancing act in creating a sustainable system that achieves one version of the truth while meeting the individual requirements of standard bodies, solution vendors, and end-users.
Core Element 2: Smarter Tools and Approaches for Prevention and Outbreak Response
“Work smarter, not harder” was a term coined by Allan F. Morgenstern when advocating his philosophy of work simplification.
One could argue that although Morgenstern was an industrial engineer he could never foresee the amount of data that could be generated from even simple processes with the data capturing tools at our disposal that have blossomed within the century from when he first coined this phrase.
Specifically, for food safety, the SQFI already advocated for Statistical Process Controls which would allow food manufactures to deploy a suite of analytical tools to reduce waste, rework, and scrap, and ultimately cut down on their workload.
Although the requirements for Statistical Process controls were walked back to just Process Controls between SQF Edition 8 and 8.1., the principle of this vision remains in Core Element 2 of the FDA’s plan of leveraging technological solutions to do in an instant what would otherwise require significant manual labor and resources to achieve.
Core Element 2 shows the FDA’s vision of leveraging technological solutions to do in an instant what would otherwise require significant manual labor and resources
With an improved traceback program in place, it stands to reason that our ability to conduct root cause analyses (RCA) will become greater, and the results from those improved RCA’s can help drive the prevention-based framework that was established when FSMA was implemented.
As technology continues to evolve and becomes more widely available across business groups, and as more data streams and tools for rapidly analyzing data become available, it will be important to understand how the industry can utilize predictive analytical tools to identify contamination events before they occur, prevent contaminated products from entering the food supply, and remove potentially contaminated products from the market.
I discussed ‘What happens when you get it wrong’ in traditional systems before, but in Safefood 360⁰, a Corrective Action could be triggered with one of the actions being a RCA performed by an authorized team member.
The results of the RCA would be fully documented in the Corrective Action, and the appropriate preventive/corrective actions could then be carried out, followed by subsequent final close out of the action.
Each streamlined Corrective Action is linked directly back to a source record enabling full traceback within a robust system.
In addition to the Corrective Action tool, predictive analytics tools provide dashboard views of the entire system in real-time.
At just a glance, you’re able to know where your compliance and risk levels are at or take action to improve them.
Core Element 2 shows a commitment from the FDA that it will pursue innovation for industry and incorporate more technology solutions, that should ultimately make life easier for food companies, but do significantly more work.
Looking back at Morgenstern’s advice, we continue to innovate our platform and enhance our services, meaning that we work smarter, so you can work harder.
Core Element 3: New Business Models and Retail Modernization
To say that the world has completely changed since COVID-19 is almost an understatement.
The industry has essentially been turned around, not only at the manufacturer level, but at the retail level and arguably faces its greatest challenges ever.
The food industry is arguably facing its greatest ever challenge
Prior to the COVID-19 pandemic research indicated that online grocery shopping would have a 20% share of consumer food spending over the next years.
Arguably, with the requirements of social distancing and the unexpected uptick of those that would typically not use such as service finding benefit and value in it, it’s likely that these numbers may well end up being significantly higher than originally estimated.
Sheltering in place was not necessarily conducive to shopping at brick and mortar retail, and although it is too soon to speak with clarity on the long term effects it will have on consumer behavior, online shopping and pick up services at least doubled.
In the traditional retail channel established, supermarkets have refrigeration units and are subject to routine 3rd party food safety audits.
This means applying oversight in these channels is still a relatively straight forward process as the variables can still be anticipated and controlled within reason.
However, what can the FDA do to ensure food safety when it comes to online grocery shopping as the proliferation of services increases?
Core Element 3 seeks to address this, but begins with an acknowledgment that it is an onerous task that faces the industry.
In this, the FDA claim their goal is to collaborate with regulatory partners and a “broad array of stakeholders” to “convene a new food business model summit to identify future courses of action to address potential food safety vulnerabilities”.
Modernizing traditional retail food safety will require a focused and considerable effort to ensure the FDA Food Code is uniformly adopted by local, state, tribal, and territorial or federal regulators and food protection programs to ensure a consistent national food regulatory policy.
Core Element 4: Food Safety Culture
Fostering a food safety culture might be last on the list, but certainly is not least.
During the COVID-19 pandemic, we saw the importance of keeping food workers safe, and we also saw how it important it was to ensure that those who were preparing food at home were safe and had an understanding of safe food handling practices.
A strong food safety culture is a prerequisite to food safety management.
This is an approach that has already been adopted and championed by the BRCGS and has grown in stature within the US.
I recall the seismic shift that rippled through the industry when the GFSI food safety benchmarked schemes hit the shores of the US.
If you were involved in food safety back then, you might recall the letters from small retailers demanding that you be certified to something called SQF.
It wasn’t until a “not to be named” large company letterhead arrived demanding you be certified to the SQF standard that anyone even decided to find out what SQF was.
Collectively as a group, we all learned pretty quickly what SQF was and today it’s probably difficult to find a food manufacturer that doesn’t know.
The food industry in the US has completely changed over the last 14 years, and with this Blueprint, the FDA is hoping to help change it even more.
In order for change to happen though, it needs to start from within the company, just as it did 14 years ago.
What does the future look like post-Blueprint?
The New Era promises to usher in an evolution of food safety practices within the US.
At its core, the FDA are wishing to work hand-in-glove with industry and learn from individual stakeholders and certification bodies and schemes as to what works best to create a singular utopian view of what an achievable and sustainable system will look like.
Whether or not this is a bridge too far and is a case of too many cooks spoiling the broth, it promises to be an exciting time and shows an ambition arguably not seen in the U.S. since the sweeping reforms issued in following Upton Sinclair’s ‘The Jungle’.
While I am not implying that the reforms are in any way as required now as they were then, it is an exciting time to witness an altruistic vision of what the future may hold that will bring benefit to everyone from the farmer to the final consumer.
If the FDA are successful in achieving this mandate in the years to come the benefits for the U.S. food system will have significant positive impacts on public health and the larger economy, and could well place the U.S. at the forefront of global regulation and oversight.
The Blueprint could well place the U.S. at the forefront of global regulation and oversight
If, on the other hand, it can only achieve parts of this ambition it could still achieve these goals or at least inspire other nations to build on these principles and reinforce the security of our global food system.
I used a metaphor at the start of this conversation that if FSMA was the foundation, then the Blueprint is the house, but it is perhaps more apt to say that if a rising tide raises all ships, then what the FDA are seeking to build is not just a house, but a boat that will rise and embolden all other adjacent systems with it.
As the tide rises Safefood 360° will continue to innovate and ensure we offer the best-in-class software solutions to maintain compliance with all major global and retailer technical standards.
If you would like to see what this means and how we can help you meet and maintain these FDA obligations in the years to come, whatever they may be, please leave a comment below, reach out to me directly, or simply just request a demo.

Understanding the difference between PRP, OPRP & CCP – An introduction
in blog, Food Safety KnowledgeLearning from the past to avoid future food safety mistakes
in blog, Food Safety KnowledgeRecently, I set myself an ambitious goal and decided to hike Hadrian’s Wall.
If you don’t know what that means, in 122 AD Hadrian, the then Emperor of Rome, set out to draw a concrete line in his empire and cement his legacy by building a wall that would mark the edge of the Roman empire.
Stretching from Wallsend in the east of Newcastle-upon-Tyne to Bowness-on-Solway, less than a mile south of the Scottish border and north of Carlisle in the North West, the wall is approximately 84 miles long (or 135 km).
With two friends, we endeavoured to not use smartphones and relying on maps and road markings hidden behind bushes we could sight, as well as the directions of the (very few) locals we happened to stumble across who were out walking their dogs, or laughing at the naive tourists.
All was going as well as could be expected, until the end of day 2, when I slipped in some mud and ruined my shoes.
I am quite an avid runner, but a novice hiker, and didn’t anticipate this.
Running in a race is an easy enough task once you crack the routine; wake up in the morning, check the weather, dress for what may come, set out for the day, and hopefully, come home successful and tired.
Anticipating potential pitfalls (literally) and how to bounce back from them over a 5-day trek in the remote countryside, is another matter I had no frame of reference for, so failed to properly prepare for.
As I stumbled out of the mudpit, I was unable to dry my shoes thoroughly at the next pitstop, and my feet began to blister on the 3rd day.
By the 4th and 5th, as we came in to the home stretch, my brothers-in-arms who I had set out with where long since ahead of me and enjoying a well-achieved finishing ale and cider respectively, I stumbled into the last 10k of the walk, hobbling under an immense pain, but with a determined ambition to finish.
In this instance, I admonished myself for not being adequately prepared, and belittled my own success, realising that although I was approaching the finish line, I’d made things immeasurably more difficult for myself than my friends who were more used to hiking and had prepared appropriately, with adequate and purpose-built clothing, an expectation of (real) pitfalls, and who were well scarred with previous travails, callouses, corns, and all.
While doing so, I thought of all things to take my mind from the pain, and my mind travelled back to a conversation I had with a recently appointed QA manager at the European Food Sure earlier this year.
They mentioned that their current method of working with SharePoint and excel was causing significant pain and undue stress on their teams and that most of their working week was being spent chasing materials and completing paperwork rather than leading their staff and systems in the direction that matched the company’s growth targets and strategy.
I drew the parallel between my current situation, not having researched and prepared as well as my companions, to hers, not having the appropriate toolset to make her life easier.
What I know from working with these companies on a daily basis transitioning from traditional systems to fully cloud-based Food Safety Management Systems, is that manual systems by their very nature require dramatically increased inputs in order to maintain them, never mind growing them to accommodate a business as it scales.
Without great quality data and experience we are completely blind when we are faced with decisions
The realization appeared, almost like the final pub did over the brow of an upwards hill (the ignominy of the finish line), that without great quality data and experience, we are completely blind when we are faced with decisions that need to be made.
The importance of great data
Imagine a scenario where you join a company that have no records, have never heard of HACCP, and have just focused on production, without ever realizing the importance of demonstrating their compliance.
You have been tasked with righting the ship and prepping the staff to be audit ready.
Where do you start?
Do you begin with compiling data for non-conformances, failed calibrations, risk analysis of suppliers, or even the individual output of each employee?
If you focus on one particular aspect, how best to start determining the most appropriate way to make educated decisions about the future?
Without any data, no matter which you choose, you are equally as blind and operating on guesswork and assumptions. You may get it right and start with the most significant resource drain, but you’re equally as likely, if not more so, to get it just as wrong (a topic previously covered in a former blog ‘What happens when you get it wrong’).
Good data and strong tools are the best friends you need when making any decision, whether it’s for a hike or for determining the quality and legal compliance of a Fortune 500 at a Business Group level.
Combining these two for any scenario reduces a lot of pain and allows for fragmented and disparate individual elements or areas of assessment to be brought together to reveal a more encompassing big picture.
Cloud-based Food Safety Management Systems allow you to achieve this and bring all of your food safety data into one organized, coherent, and structured system which is always accessible and retrievable.
The struggle of maintaining applications versus data retrieval
Say you get the system off the ground, everything works as it should and you have full oversight.
With everything in hand, the business decides to progress and bring to market new products.
This means more production lines, perhaps more sites, these you know less well, and you may need to re-conceptualize the wheel again to accommodate where they fit in to your plans and structure, and how you can effectively support their safe operation with your current commitments.
There are only so many hours in a given day and you have to focus on the operation of everything you have already created.
So, you do what can reasonably be expected and bring in help.
This help doesn’t know the system as well as you, nor too may they be as inclined to put in the same level of elbow grease that you are.
This system is your baby, your creation that you know inside-out and have given life to, and while new hands will help operate it, they have their own viewpoints, inputs and contributions that may affect their system.
With the best intent, as your eye focuses only on your own tasks you are responsible for, creep may set in and exponentially, over time, the systems may become different versions of themselves, appearing similar on a surface level, but truly an uncanny valley under the hood.
The natural tendency for any manual management system is towards disorganization, disintegration and ultimately failure.
The natural tendency for any manual management system is towards disorganization, disintegration and ultimately failure
If tasks are not completed as stated, the system loses integrity, and the more it depends on human labor and manual work, the quicker it slips into disrepair, and inevitable ruin.
Internal audits are often planned and scheduled on a grid system which defines what is to be audited, who will perform it, when it is to be conducted, and against what standard or checklist.
Planning this alone requires a lot of input, and implementing its application requires more in oversight at a higher level, which too often requires the most domain knowledgeable staff.
Stepping back a moment, before this is even applied, it requires a human to assess the plan, and if they fail to do so appropriately, or are late in doing so, the system has already begun to crack and show fault.
Of course, this ignores that the output derived will then yield nonconformances, which need to be notified, assigned, reviewed, and corrected.
Getting great data requires a great system where these problems do not occur in systems which are by their nature, pre-disposed to faults and failures, or at the very least mitigated against so their impact is minimal.
How food safety systems use technology to get data
When I want to travel between two points, I know that I always have the option of a plane, train, automobile or other.
Likewise, I know that in these situations for QA’s and Technical Directors and others, that when they want to future-proof their systems, they have the option of utilizing a system like Safefood 360°.
We already work with many of the word’s leading food businesses around the globe that are compliant with FSMA, GFSI, BRC, and what can seem to be an almost innumerable amount of other local legislative and retailer standards like Tesco, Costco, Coles and many others.
These systems work with us not because we’re a panacea for their woes, but because we fundamentally understand what is important to them in the first instance, the continuous refinement and improvement of their systems, and give them the tools to accelerate and build towards this.
Safefood 360° offers best-in-class BI tools that allow you to immediately see at a bird’s-eye view what is going on in your system in all places, in real-time.
see at a bird’s-eye view what is going on in your system in all places, in real-time
This means no more late nights scratching your head over an excel sheet or spending more time than necessary constantly referring between two screens wondering how two facilities that are supposed to be operating the same systems and standards, with the same training and support, are yielding vastly different results.
It means no more travelling to these sites, and constantly greasing the wheel and assessing, retraining, and re-assessing to make sure it sticks and is embodied in the culture of its operators.
We do all this while we conduct the heavy lifting for you.
Ensuring that your system is built, to your own perfect standards, so that you can continue to focus on what’s important to you, while your system continues to work to the ideal standard you envision.
Appreciating the every day
We live in an age of technology where we take most things for granted.
I can drive to the office, fly to meetings, or take a boat down the canals of a conference in Amsterdam, yet I chose to walk across a fly-over countryside just to know that I could.
A journey that took <90 minutes on a train took 5 days, and 3 more of healing afterwards.
Was it any more rewarding? Arguably, I got more satisfaction knowing that I could do it, but was any more difficult? Exponentially!
Would I do it again? Never, once really was enough and the opportunity cost of 7 days was too noticeable to give up again.
Technology allows us to unshackle ourselves and accelerate production at an unthinkable level from 10-15 years ago. Yet, too often do we marginalize it in the face of compulsory tasks and only ever think it can be used at a macro level because we think it might be too much work to set up, never mind that the reason it seems too much work, is because we are inundated with consuming tasks from a broken system in the first instance.
When we focus on solving the every day, we can really appreciate it more and give ourselves the time and luxury to focus on the future and the next steps.
I came home after the hike and although I couldn’t walk, I embraced my laptop as it allowed me to keep in touch with the office while my foot healed and saved me from having to do any more walking or commuting to the office, making my life a lot easier.
The good news is, you don’t need to fly to England and hike for a week to experience the same.
Let us help have the technology work for you, and show you how Safefood 360° can be applied to help make your life easier today.
Food Fraud: Challenges and Strategies
in blog, Food Safety KnowledgeFood fraud has become an ever-growing problem that affects products and businesses across the supply chain, and in the UK alone, it has been said to have cost industry over £11 billion, and as much as $40 billion in the USA.
Food fraud occurs when products are deliberately mislabeled or misrepresented, diluted, tampered with, substituted, counterfeited, or subjected to unapproved enhancements.
The pronounced effect of this is that food fraud is a complex commercial and supply chain issue that is not something that can be solved by a Quality or Technical department alone.
A brief history of fraud
There are many reasons why fraud occurs from a desire to increase profit margins or the product itself being cheap and easy to copy, to unsatisfied market demands, and difficulties in detecting and proving the providence of a good, which in some cases can make it easier to carry out.
‘Horsegate’, in 2013, was one of the most publicized cases that pushed food fraud to the forefront of media attention in recent years.
When testing on frozen beef burgers first revealed undeclared equine DNA, it soon became evident that affected products had been disseminated throughout the supply chain and resulted in several European countries being affected in the fallout.
This fallout was significant, yet it was not the first, nor is it likely to be the last, instance of food fraud on a significant scale that garners worldwide attention.
In separate instances, in both 2007 and 2008, melamine originating from China was found to have a presence in pet food and baby formula, highlighting what can happen when the motivation of profit exceeds product integrity.
When the motivation of profit exceeds product integrity… surely no economic gain can be worth such an outcome.
Included to show adequate protein content after water had been added to raw milk to increase its volume, the contamination with baby formula led to many illnesses and the deaths of 6 infants, surely no economic gain be worth such an outcome.
What motivates food fraud?
Recent scandals such as these have further highlighted the need for companies to provide mitigation strategies within their organizations and across their supply chain to help reduce food fraud.
Typically, it may be best practice to conduct a vulnerability risk assessment of the supply chain, which usually includes looking at 3 main aspects: Opportunities, Motivations, and Mitigations.
For any given food product or ingredient, the nature of its composition, qualities, production process and supply chain, as well as its geographic origin, determine the opportunity for fraud.
For example, it is generally easier to commit fraud on liquid products and complex foods with more ingredients than simpler foods with fewer ingredients.
When assessing the opportunity of food fraud occurring to the product, questions that should be asked include:
- How complex is the supply chain of the product?
- How robust is the security along the supply chain?
- How easily can adulteration be detected?
- What detection methods are available?
Perhaps, Professor Chris Elliot of Queen’s University Belfast, Ireland, put this best when he remarked that “every time you have a transaction, there’s another opportunity to cheat.”
“Every time you have a transaction, there’s another opportunity to cheat.”
At its core, what this means is that food fraud can potentially occur at any stage in the food supply chain, and we must be vigilant at all times to its potentiality.
Once an ‘opportunity’ is identified throughout the chain that fraud may occur, it is equally as important to consider ‘why’ this might occur in the first instance.
Once an ‘opportunity’ is identified throughout the chain that fraud may occur, it is equally as important to consider ‘why’ this might occur in the first instance.
Once the motivation for fraud can be considered, it can determine at which stage fraud will more likely occur and what additional steps can be taken to mitigate against its occurrence.
Asking yourself the questions, how would you describe the economic conditions of your direct suppliers and how would you rate the corruption level of your suppliers, will be a step forward in helping to identify the motivation of food fraud through your supply chain.
Basic economic motivation can be classified as one of the main reasons for food fraud and this can be driven by revenue maximization or cost minimization.
Economic motivation can be driven by revenue maximization or cost minimization.
Once any motivation has been identified you need to put in place mitigation strategies to help prevent this potential fraud from occurring.
Mitigation strategies for food fraud
The best prevention against food fraud is to anticipate the reasons why it may occur, both internal and external, and to mitigate against them.
A necessity for a food business is to fully understand each of the stakeholders in the chain involved in producing, storing, and distributing their food ingredients.
A common struggle for companies is Supply Chain Mapping, which is extremely relevant in paving the way to help reduce food fraud.
Effective procurement, robust supplier approval, and supplier management are core elements to minimize any potential supply chain risks.
Not only is the management of your supply chain vital, but a company also needs to start from within their facility by looking at their food safety management system, management itself, and employees, which all play an important role in reducing the risk of food fraud.
A company needs to start from within their facility.
It’s important to consider employee integrity screening and an ethical code of conduct, and to not forget that employees and staff are at the forefront of production every day.
Those at the front line need to be comfortable if they see anything abnormal happening, i.e., potential food fraud, that you as a company have policies in place that protect them if they come forward to discuss.
Being able to track your raw material right from the beginning of its life and knowing the supply chain between then and when it arrives to you is also paramount, so an effective and strong traceability and mass balance system should be in place as well.
A robust supplier approval process is key, and it is important to really think about what standards you want your supplier to have.
This was previously discussed in one of our more recent blogs (What criteria can you use to risk assess your suppliers?), and it should take into consideration their ethical code of conduct.
Going further, you may also want to consider if there are any whistleblower policies in place, even if this means asking questions to staff during an audit to help assess whether they may think any strange activities may be happening.
Lastly, along with the above, we cannot forget raw material monitoring and testing (with a representative sample) is an important aspect in the fight against potential food fraud, although this may not always be easy.
Testing may not always be easy, and can be expensive, time-consuming and a large representative sample would be required to just rely on this strategy alone.
Food fraud in the future
I believe it is safe to say at this moment there are industry-wide challenges when it comes to the detection of potential food fraud.
It is certain that food fraud is an ever-growing concern for all those involved in the food industry, and can occur at multiple points and from multiple sources, with a wide array of motivations.
Widespread opportunity, adulteration and deception, as well as the ever-growing complexity of the supply chain, makes our lives somewhat more difficult, but equally as rewarding.
Widespread opportunity makes our lives somewhat more difficult, but equally as rewarding.
Those committing food fraud are criminals and deception is their main goal, so they will do everything they can to hide that this is happening, and therefore, this will make it more challenging.
We need to be vigilant and determined within the food industry to fight against this fraud and we need to learn from previous public food fraud cases, I would imagine that no one would want to face the repercussions of such a scandal to their company and damaging effects to consumers.
An article I read previously stated ‘Criminals are smarter than Salmonella’, and at this moment in the food industry, this is probably something many could agree with.
One mitigation strategy alone will not help to combat this fight, but rather a multi-faceted proactive approach, transparent systems, and constant vigilance is required at all times.
7 most unsafe fictional foods to produce (from a food safety compliance perspective)
in blog, Food Safety KnowledgeHow Safefood 360° helps with the changing Canadian Food Safety regulations
in blog, Food Safety Knowledge, Product UpdatesAs of today, food businesses manufacturing in or exporting to Canada need to be compliant with the new Safe Food For Canadians Act (SFCA) and the Safe Food For Canadians Regulations (SFCR). These new additions to the Canadian food landscape replace 14 sets of regulations which had previously been the standard for compliance.
What are the Safe Food for Canadians Act and Safe Food for Canadians Regulations
Similar to the enforcement of the FSMA regulations in the United States, the increasing complexities of modern food production and supply chains have necessitated this demand which will see the government of Canada’s food safety focus shifting even further towards the prevention of foodborne illness.
Its effect on government sees the consolidation of the Fish Inspection Act, the Canada Agricultural Products Act, the Meat Inspection Act, and the food provisions of the Consumer Packaging and Labelling Act.
What this means for Canadian consumers, in summary, is that:
- Food is as safe as possible for Canadian consumers
- Consumer protection is enhanced by targeting unsafe practices
- Tougher penalties will be enforced by activities that put health and safety at risk
- Enhanced control over imports are now granted
- Establishment of a more consistent inspection regime across all food commodities
- Strengthening of food traceability
How does Safefood 360° comply with the SFCA and SFCR?
As the SFCA and SFCR bring Canada closer in line with other regulations such as FSMA in terms of its reach and enforcement, we are pleased to announce that Safefood 360° is compatible with the new changes out-of-the box.
This means that users operating Safefood 360° at their facilities can operate securely in the knowledge that their goods are compliant with the changes in Canadian law.
For a more comprehensive understanding of where your business stands in regards to compliance with the changes in Canada you should compare your business against the full regulations.
Safefood 360° is committed to ensuring our users are compliant with all major retailer and technical standards globally.
Please let us know your feedback and thoughts, and should you require any further assistance with complying with these changes, our support team is available through the software to assist you.
Facing and Controlling Cross-Contamination in Food Processing
in blog, Food Safety KnowledgeCross-contamination can occur at virtually any stage in the flow of food processing, and prevention is crucial.
Understanding where and how it can happen makes prevention easier, and the four most common routes of cross-contamination are outlined below:
1. One of the main means of cross-contamination is actually the individual who handles the food.
For instance, food can become contaminated if a worker who was dealing with raw chicken earlier didn’t effectively wash his/her hands prior to handling ready-to-eat products or if they forgot to put on gloves, or perhaps they are wearing a soiled uniform.
2. Another route of cross-contamination is unclean equipment where an uncontaminated meal can come into contact with an unhygienic utensil, part of a device or maybe an unclear work area.
For instance; if the raw material receiving truck driver did not wash down their truck before carrying the materials, then there is a chance that the raw material is contaminated.
3. Cross-contamination can also occur due to direct transfer between various foods, say when a meal drips contaminated juices onto another meal. The most common example here is the storage of uncooked meat besides ready-to-eat food products.
4. Transfer of food allergens from an allergenic food to non-allergenic food is also one of the means of cross-contamination.
Mislabeling and mishandling of food products are justifications for allergen cross-contamination.
It is not too difficult to imagine a scenario where the worker who is transferring the food from the temporary storage room to the packaging area takes a detour to the processing area and unintentionally walks by the wheat production area.
Food allergies are a significant problem for individuals who have to contend with them.
Not only this, but the company might face lawsuits and gain a bad reputation due to their mishandlings of food products.
Now that we have established the most common means of cross-contamination, let’s look at the different ways by which you can successfully avoid them.
Personal Hygiene
A well-designed personal hygiene program is a must when it comes to avoiding cross-contamination.
It is highly likely that food handlers will transmit foreign material from their skin, hand, or hair without a proper hygiene program in place.
The following program will highlight different best practices in food handling such as handwashing, glove use, and hand care.
The aim is to reduce the possibility of food cross-contamination through employee awareness.
This prerequisite program should also include personal habits such as bathing, clean clothing (and the importance of a clean uniform), and the use of hair and beard nets at all times.
The health of employees is another factor that influences cross-contamination.
An unwell employee should be removed from any area where there is a moderate likelihood of food or related ingredients becoming infected.
It is important to know if employees are healthy or if they have any disease or illness that could contaminate your food product.
Therefore, regular inspection and medical screening prior to employment should be conducted and a record of illness and injury should also be maintained.
Workers’ hands must be washed thoroughly and every so often while handling the food.
For instance, after handling raw meat, seafood or after using the restroom, they must remember to wash their hands, and after washing their hands it is vital to ensure that they are dried properly by using paper towels.
Finally, they should sanitize their hands prior to returning to work.
Each year, millions of foodborne illness cases occur all over the world.
Therefore, it is critical that all involved parties focus on ensuring safe food handling.
Employee training is key as it will encourage them to follow everyday sanitation rules and thereby reduce the chance of people becoming ill due to cross-contamination.
The training should include basic food safety practices such as personal cleanliness and good sanitation practices to prevent food product contamination.
Posters demonstrating best practices can be displayed where possible as additional support to the training.
Color Coding
The most straightforward way to put a stop to contamination and distinguish between workstations is color coding.
It is a well-known fact that the tools used for raw meat should not touch the end product.
This helps to control the risk of pathogens such as Listeria Monocytogenes, E. Coli, Salmonella and so on (Learn more about pathogens in our datasheet library).
Another advantage of changing tools for different processes is to control cross-contamination of common allergens.
In both cases, if a food manufacturing facility uses color coding to distinguish between the tools and equipment it will help to mitigate cross-contamination and support the facilities with maintaining Good Manufacturing Practices.
Identification of appropriate areas for storage, cleaning, and sanitization is also much easier with color coding tools and locations.
Occasionally, food processing facilities use unique color coding.
Food facilities can determine the colors which best suit the company’s objectives, processes, product types, and facility design.
For example, a peanut processor may select white for direct contact while a processor of milk powder might choose a different color such as green or red.
The color coding system will only work if it is properly communicated to the employees.
Clear guidance should be provided to the employees outlining the importance of the program and exactly how it should function.
The procedure should also explain the steps to take in the event of a nonconformance.
Process Flow
A cross-contamination prevention plan should also consider how the process moves across the company.
From the starting point, i.e., the receipt of the raw material, to the finished product, the process should be evaluated to understand how ingredients come into the facility and how they will be processed.
This will help to determine the crossing of product and probable points of cross-contamination.
The solution to prevent cross-contamination could be to move products in sealed packages/containers and confirm that no leakage is present.
If it is not possible or allergens are of concern, then sanitization of the area is the key.
It is also a good practice to complete the production of non-allergenic foods first and then allergens at the end of the day.
The production line should be sanitized following the last batch.
Where this is not possible, sanitization processes need to be in place in between the change of production.
Cleaning
Cross-contamination will occur if the food product comes into contact with insufficiently cleaned surfaces such as equipment, clothes, and utensils.
The efficiency of cleaning must be inspected on a regular basis and the cleaning policies should outline the systematic procedure, i.e., high-risk areas should be cleaned first.
The most dangerous source of microorganisms in a food production facility are the floor or drain.
Cleaning and sanitization of floors and drains should be repeated at the end of each production day along with precautionary measures for the duration of production to minimize the microbial growth and food safety risk.
Cross-contamination between different plant areas can be managed by using the methodical sanitation program, which you can read more about in the Validation of Cleaning Programs whitepaper.
Ahead of using cleaning chemicals and sanitizers, it is of the utmost importance that employees read and understand labels and the material safety data sheet as well as understand how to use the appropriate personal protective equipment.
They should also understand how to use signs, adhere to procedures, and be careful while moving on the wet floor.
Cleaning is not only limited to food contact surfaces; as mentioned previously in this article, clothes are a known source of cross-contamination.
Bacteria or food particles can be transported from one place to another by means of dirty clothing and in order to stop this, clothes should be changed when moving from one production area to another.
This is especially crucial while working with high-risk foods or allergens.
A plan to eliminate cross-contamination can be put together by combining all of these different pieces. However, it still does not mean that nothing will go wrong during daily production.
The strategy of cross-contamination prevention needs to be reviewed periodically, and it may be wise to consider doing so on at least an annual basis.
The food facility will need to change its approach every time a substantial change occurs, i.e., if a new product is developed or supplier is changed.
A thorough plan that is rigorously enforced will help identify and tackle any weaknesses in the process flow, containing and resolving issues within the facility and preventing cross-contaminated products from reaching consumers.


