7 Ways Compliance Management Platforms Improve Operational Efficiency of Food Manufacturers
Seven Ways Compliance Management Platforms Improve Operational Efficiency of Food Manufacturers Food is life
Seven Ways Compliance Management Platforms Improve Operational Efficiency of Food Manufacturers Food is life
From leafy greens to pet food and everything in between, too often these days it seems as though recalls and issues for food safety issues are growing in frequency.
One may argue that as we are becoming more aware of these events in this interconnected ‘Information of Things’ era our hyper-vigilance is moving us towards halcyon days removed from ‘Horsegate’, Melamine outbreaks and other nefarious criminal antics.
As the global food supply chain grows in complexity so too does the pressure on the individual agents to prove they are compliant in a climate where there is an increasing dependence on others within the chain to source partially processed products and raw materials.
A kaleidoscopic amount of legislation advances and technical standards that place greater emphasis on culture and practices that would not have been prominent in the QM’s mind not even 10 years ago, only emphasize the strain that food businesses today face, under pressure from a growing number of regulations, too often under dwindling resources and time to solve them.
External pressures from suppliers and customers might also increase the burden of emphasis as businesses often have to demonstrate a ready-made solution that actively shows they are composing themselves with honesty and integrity, while now being accountable for their own supplier’s supplier.
With so many contrasting pieces of the puzzle moving independent of each other, it can be understandable if a Technical or Quality Manager is at a loss for where to start when considering how to build and structure a robust compliance program for food safety.
When David fought Goliath, you could argue that Goliath had more to lose.
Similarly, when a small business and a large global operator consider the need for reviewing and assessing their compliance program, too often by nature of having more output, and thus, ‘more’ to lose, the ‘Goliath’ is more likely to have a forward-thinking and established system for tackling the issue.
Typically, this is because the larger business often has more resources in both capital and expertise to dedicate towards the issues.
Equally, for small or medium-sized operations budgets are tighter and any errors can incur a disproportionate amount of financial pain.
Regardless of size, failures in compliance or inadequate structures cause significant disruption, damage, downtime, and potentially even death in the worst-case scenario.
In such situations, it may seem a case-by-case basis may not be adequate and can expose the business to more significant duress, as well as high direct and indirect costs in the longer term than if they had an established compliance program.
However, what can be assumed to be true is that the cost of quality will be felt by the business, regardless of its proportion to profit or revenues, so it is best to act as it can only benefit a business in the long term.
Too often the ‘agile’ nature of smaller or disruptive businesses can often be a hindrance as responsible parties wear a multitude of hats.
In circumstances where ‘management’ is responsible for all areas of the business from product conception to customer relationships, as well as production itself, the limitations of the businesses are quickly revealed.
In smaller businesses, it may even become harder for management to step back and be analytical towards the needs of the business, as they are too often involved in the day-to-day operations, and can potentially lose sight of the ‘bigger picture’, so they can often end up focusing only on what they know.
This situation can often drive many small to medium business owners and managers to ignore a robust quality system in favor of legislated or minimal testing regimes.
As with starting any journey or puzzle, it may seem daunting in its initial assessment, however, there are some simple considerations a business may opt for in order to help it decide on its strategy for enhancing its robust compliance program.
Whether you are starting to build a compliance program from a foundational level or inheriting a legacy system that is functional and in need of modernization, it is important to inwardly assess and understand the resources at your disposal, and how available those resources are as you begin your compliance journey.
Ensuring senior management commitment is, therefore, a foundational step that must be guaranteed before any assessment begins as this will help to ensure it is comprehensive and fair.
Ensuring senior management commitment is a foundational step that must be guaranteed
The first requirement under BRC Global Standard Food Safety Issue 8 requires Senior Management Commitment and Continual Improvement where ‘clear objectives are defined to maintain and improve the safety, legality, and quality of products manufactured’, which are monitored and the results of which are reported ‘at least quarterly to site senior management’
Likewise, in the SQF System Elements, we find a very similar clause in 2.1 ‘Senior site management shall prepare and implement a policy statement that outlines…the sites commitment to safe food…the site’s commitment to establish and review food safety objectives.’
Senior management commitment and support can manifest in a multitude of ways, but at its core, it should contain a willingness to hold those responsible for activities accountable in the event of failure, be proactive and not reactive, and include frequent and transparent staff meetings, briefings and reviews of performance indicators like audits and customer reviews or complaints.
Senior management commitment and support can manifest in a multitude of ways, but at its core it should contain a willingness to hold those responsible for activities accountable in the event of failure
This commitment reflects that “the starting point for an effective food safety plan is the commitment of senior management to the development of an all-encompassing policy to guide the activities that collectively ensure food safety” that emphasizes food safety compliance as a “cross-functional responsibility which includes activities that may draw on many departments” (BRC Global Food Standard Issue 8, Page 3-4)
| Effective food safety requires commitment from |
|---|
| Technical Departments |
| Production Operations |
| Engineering |
| Distribution Management |
| Raw Materials Procurement |
| Customer Feedback |
| Human resources (Training) |
A businesses’ shape is determined by a number of factors such as what products are made, the manufacturing processes and technologies used, how many people are employed and their roles, not to mention the cash flow situation and how the products are distributed and what the customer base is.
A small business with a few employees performing multiple roles selling fresh, uncooked, manually-packed products to a market in an area of predominantly elderly people is a substantially different prospect to the company that provides the same products, packed mechanically with a larger manufacturing base selling into nursing homes.
it is vital to understand the paramters of your business and the atmosphere in which you exist
Remember, companies with greater financial resources have more options than cash vulnerable companies, and this can often affect the shape of your overall program.
Before any outward action can be taken with regard to establishing your programs, it is vital to understand the parameters of your business and the atmosphere in which you exist.
The reality of business is that there are standards imposed from external sources to ensure that health and consumer-related issues are addressed. These standards may come from governing bodies, association memberships, customer requirements, or even ethical standards.
Amongst others, these issues include:
Aligning food safety management compliance with general business compliance for greater integration with compliance functions may cause pressure to ensure you are mindful of processes, hazards, regulations, contractor risk, product type & industry sector that can give SME’s a headspin and get them looking for help.
the pressure to ensure you are mindful of the realities of testing can give SME’s a headspin and looking for help
The key requirement of any system is that it proves compliance, not only for food safety but for quality or any claims you make about your product.
Equally, at this stage, you might also begin to consider the internal knowledge gaps that may exist and what steps can be taken to remedy these.
Particularly for smaller businesses, if this is a new undertaking where you have no internal stakeholders with prior experience or direct knowledge of the task ahead, it may be wise to consider seeking external guidance to assist you.
When considering opting for external validation and help there are different options available to consider.
NB: Choosing a consultant is not a one size fits all approach
In general, however, they can be employed to assist in interpreting results and standards and how they pertain to your specific case.
Although sometimes perceived to be expensive, and ask for many things to be changed, SME’s may use them to “handball” responsibility or in an attempt to get “free advice” during the quoting process.
Choosing a consultant is not a one size fits all approach as each has a different background and unique approach.
Take some time to choose the one that will gel or fit with your business and needs.
NB: When employing a consultant it is wise to consider that if the consultant is unscrupulous that they may be purely economically motivated and they what may be the best outcome for you, i.e., a speedy resolution, may not be their motivation as they seek to extend projects for their own gain.
Assembling the plan and ensuring it is structured correctly might seem the most daunting aspect before you started this journey, but assuming a thorough assessment of the above and proactive steps have been taken in researching and seeking external assistance if required, you may find the pieces of this jigsaw clicking together faster than assumed.
One of the key elements of a robust compliance management system is to ensure that it is appropriate and viable in the long-term and is not dependent on any one specific stakeholder for success or failure.
This means that success or failure is not dependent on any one agent and that there are appropriate redundancies in the business should one stakeholder or operator not follow compliance to ensure the system yields success in spite of this.
Determining who is accountable for the compliance program does not necessarily mean that some stakeholders may have more input or value than others (such as a PCQI), but rather a successful compliance program will have investment across the board and give each individual agency to ensure its success.
Fostering a successful and invested culture within the organization is one of the more modern elements of a successful system that should be endeavored by all organizations, large and small.
In Europe, BRC Global Food Safety Issue 8 includes a new section on culture, and it is a sentiment being reflected in North America as well.
More and more, from GFSI to the FDA regulations, it can be seen that food safety culture has become a key requirement in the character for the entire food manufacturing company, and no longer simply a function of the Quality department.
Equally, when establishing an arena of culture, it needs to be established how to do this, what resources and limitations are imposed and the best avenues for doing so.
Further information on incentivizing and motivating others to ‘buy in’ to improved culture can be sought here for the FDA which advises to “ensure consideration of the role of culture as a central tenet in advancing the agency’s food safety mission” and in BRC Global Standards Issue 8, 1.1.2, which prescribes that a “site’s senior management shall define and maintain a clear plan for the development and continuing improvement of a food safety and quality culture.”
Understanding the appropriate combination of help for your business will depend on your interpretation of the scope and limitations of how your business runs (as discussed in point 1 of this article)
Once the business is structured correctly the more effort you put into making good product, rather than just making product that is viable, the fewer returns, complaints, and hassles you should ultimately have to deal with.
Structuring how your business will make decisions about its compliance program in an ideal scenario, and effectively realizing that structure in practical terms, can be a significant challenge for many businesses.
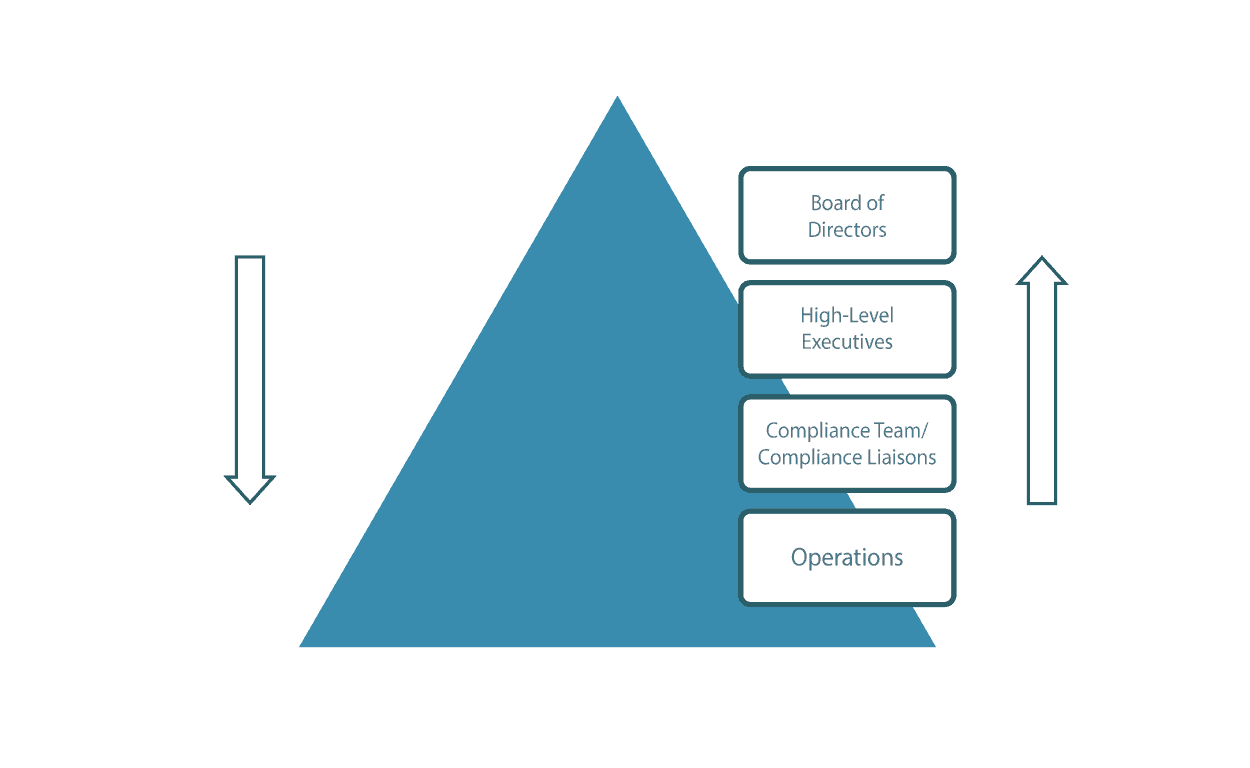
Source: Lewis – How will the company make decisions about the compliance program?
For small owner-operated businesses where the owner also works in the business, the inherent structure and needs of the business often means each person holds multiple roles.
In such situations, production might be order focused and only made when needed, and potentially, there can often be no defined technical person.
These businesses might rely on information from external sources such as consultants, regulators or industry groups and seek free information where possible.
So too may they actively seek how to lower costs and run their operations with a templated or simple system with the minimum tests required by law and customers.
When a consultant is engaged it might probably be for assistance to get by on the more problematic and technical items, and to check up with an internal audit every so often.
On the reserve of this, consider a family or partner-owned business where the owners may work in areas away from production such as sales or the office, and they know they need organization and structure, yet everyone is still busy.
Here, a person may be tasked with Quality Assurance duties, but it would rarely be a dedicated role, and it may even be necessitated by dealing with some larger clients or orders, or because you work in a high-risk industry.
Your systems and testing regimes need to be more rigorous to deal with the labeling issues, allergens, quality of suppliers and all the other points that go into providing hard evidence that your product is as good as your word.
testing regimes need to be rigorous to provide hard evidence [..] that your product is as good as your word
This is where a business structure may begin to develop organically as the business grows and scales.
When this happens, just like when a plant is grown in a garden, it would be wise to consider the structure of this and give additional support as it embeds in the business to ensure the structure is optimum in answering business needs.
Someone may be hired for the quality systems and testing side full time, a consultant may be considered, or perhaps investigate a technological system that helps offset the larger costs of full-time personnel or consultants such as Safefood 360°.
Each option comes with different benefits, but to toot our own horn for a moment, here are 7 ways smart food safety software saves you money.
As companies grow from here it becomes apparent and imperative that they often require a robust quality system, driven from management downwards with the testing backup to prove any claims made on your own or your clients’ products.
Quality programs should not only be supported, but actively driven from the very top downwards.
An owner or manager who shows the staff by their actions that they are exempt from following the full system would significantly compromise any chance of the system actually working as it is intended for the benefit and profitability of the company.
This is why a committed management team is essential to the success of any business system.
a committed management team is essential to the success of any business system
In the same vein as this, be willing to delegate and share responsibility with anyone who has an impact on the production and quality of the product.
If staff know that they speak with authority for the business or their department, they will have more agency and authority to assist in matters as and when they arise.
Stakeholders who are involved in the decision-making process are likely to feel enhanced ownership and become more effective as they have a vested interest.
Ensuring there is ownership and responsibility throughout the organization’s structure, combined with adequate resources, will give invested individuals the confidence and authority to assure the ongoing capability of the system.
invest individuals with the confidence and authority to assure ongoing capability of the system
The skill base that exists on site already is an asset that should not be overlooked as there may already be a plethora of resources internally that you may not be actively utilizing, or completely unaware of.
It can be surprising the gems you can find in your workplace, from the manager who is so engaged they treat your business with a care that they would their own, to the staff who have talents that you wouldn’t know about unless you asked.
A former colleague of mine once worked with a production manager in a small business who often spoke of his staff saying “let’s face it, they’re not university graduates”, despite the fact that when they asked, there were, in fact, four graduates out of twelve people on the production floor.
Taking an introspective look at the resources available to them, and by doing so discovering significant quality that was previously unknown to them meant that they unlocked new resources that would have otherwise remained ‘untapped’.
It very quickly transpired that this business didn’t need more people to overcome the challenges they thought they had, and actively brought their staff further into the fold and become more involved with the business output.
Ultimately, giving your team more agency into your processes should yield dividends beyond what you can imagine as people become more involved, and ultimately more fulfilled with their professional output.
Once a compliance program has moved from the page to become a realized system with an active and involved workforce, the hard work has mostly been done, but it is not the final stage.
Work and product should now be created at a rate that has fewer issues, while the need for rework and product scrap all should reduce in correlation with this.
Hopefully, this should mean a finished output of greater time efficiencies and more yield in cash-flow benefits on the bottom line.
Another intangible benefit that should accrue, but which is much harder to define, is that as your output and quality grow, the market should take notice and customers, suppliers, and even competitors will begin to regard your organization’s products in a higher light and better esteem.
Orders and income will follow suit and, hopefully, you will supply to more and more customers.
Obviously, at this stage even though it should go without saying, we’ll say it anyway, do not compromise quality simply for the sake of profit.
Do not compromise quality simply for the sake of profit
Take the time to listen to your customers and pay attention to your quality data.
Improve your quality = Improve your business = Continue reaping the rewards!
Listening to your customers may just provide you with the valuable insight required to inspire change.
Change always happens and a system is a living thing.
As your business system conditions change, your system MUST change to keep up.
Improve your quality = Improve your Business = Reap the rewards
A robust compliance system will continually evolve to meet the ever-changing demands and requirements of global food safety concerns.
It is, therefore, an important and necessary step to ensure that the appropriate people are appointed and supported with the resources to ensure effective management and continuous improvement of your program.
Any system that is rigid and uncompromising in its ability to change is likely doomed to inevitable failure as waste, downtime, and error in allocating resources will invariably appear from and detract from what good looks like.
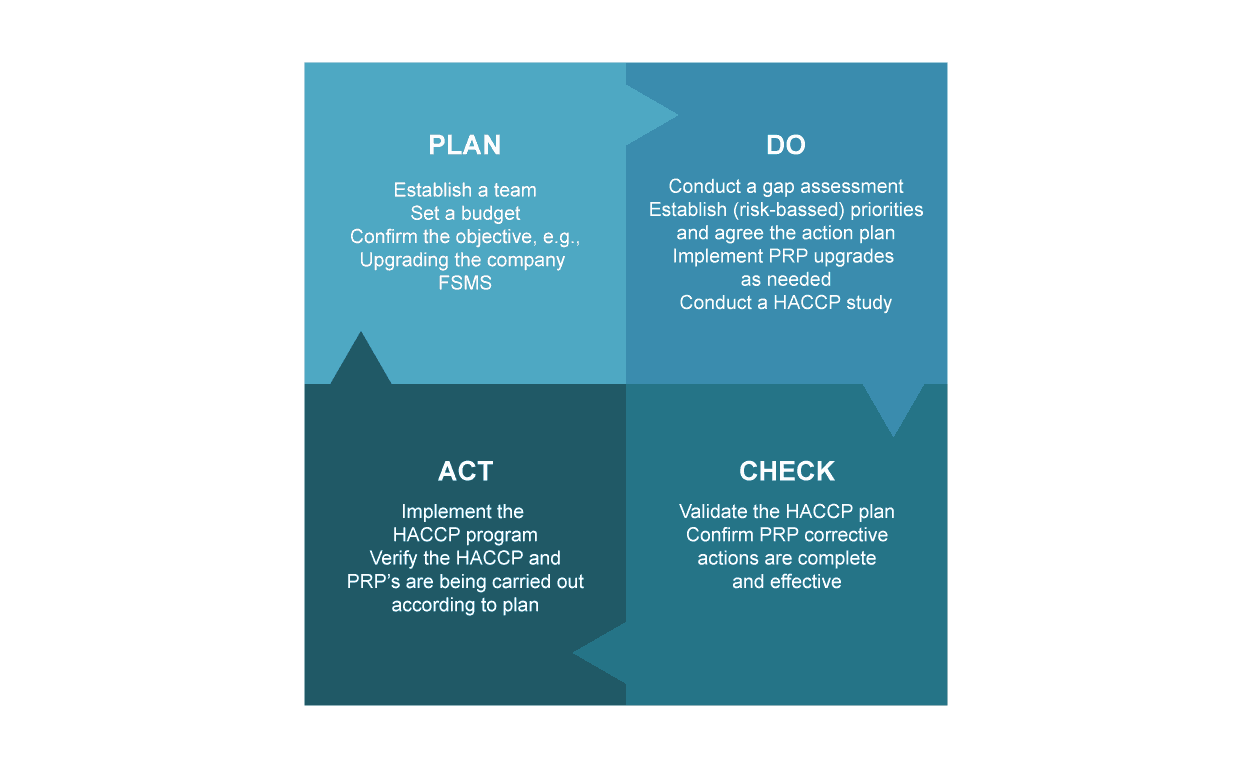
Source: Deming Plan-Do-Check-Act / The Deming Circle
Following a system that incorporates a feedback loop such as Deming’s ‘Plan-Do-Check-Act’ will establish a system of measuring and analysis, which can create a looping benefit as it allows stages for reinforcing and strengthen your programs following successful critique of their success, and their failures.
Additionally, a well-structured GFSI plan should be incorporated across the business. Achieving the initial certification is simply the first hurdle. Once you get past that, maintaining that certification is a group effort that will require effort from your entire staff.
It will likely always be easier to set up this process of continuous improvement at an early stage, then review your business and all its programs at least once a year (more if you’re growing fast).
“Applying a continuous improvement mindset towards achieving a world-class program will enable food businesses to consistently meet both their obligations to consumers and their food safety regulatory requirements. This will result in a live and vibrant food safety culture operating 24/7 throughout the food supply chain”. (Wallace, Sperber, Mortimore, ‘Cost of Quality’ Food Safety for the 21st Century, Page 161).
Guaranteeing and incorporating senior management commitment from an early stage will smooth the transition to embedding a robust compliance system that will stand the rigors of the different challenges that will appear.
Ensuring this commitment is solidified and ongoing will help this system to stand the test of time.
Food safety is ongoing and nonstop.
It is never ‘done’, ‘complete’, ‘finished’ so there is no endpoint.
Really, vigilance is the final element missing and we need to be aware of this to avoid myopia or rot setting in.
Staff training, awareness, briefings, etc., all play a part as you define the culture of a business and allow you to create a circular system that feeds communication back into the system.
A robust compliance program is stimulated internally by the need to change and self-actualization, to be better.
If an organization only waits for external factors to encourage change it is likely that they will become reactive to each concern or issue as it arises and lose control of their narrative on food safety.
Building a successful company in and of itself is an arduous task.
Building a successful company with a proper food safety culture is nothing short of Herculean.
Safefood 360° CEO George Howlett recently presented a webinar in collaboration with the International Food Safety and Quality Network (IFSQN) on pragmatic steps that can be considered when building a Risk & Compliance model for Food Safety Management Systems.
The recent publication of the ISO 22000 standard earlier this year has introduced changes to how Food Safety Management standards can look.
In general, this means a trend is becoming prevalent of food safety systems that are genuinely based on risk throughout, from top level to the smallest activity and task that might be conducted in a business.
This is also occurring with a greater alignment in the approach businesses take in identifying risk and opportunities for improvement.
This webinar is presented below in full and examines the methods and models for measuring Compliance and Risk across food safety management systems.
This includes the appropriate methods for single site and corporate environments, and also address the difference between measuring compliance and measuring risk, with particular focus on ISO 22000 and FSSC 22000.
To learn more about how Safefood 360° can be implemented in your business to assist with your food safety management system, contact us below for a product demonstration.