Improvements to Assessment Workflows in Supplier Control
We have added three new stages to the Assessment Workflow in the Supplier Control module.
These changes will better align the Assessment Workflows with the requirements of FSMA and other international standards.
The new and improved Assessment Workflow will apply to both Supplier Assessments and Material Assessments, which means both Assessments will be conducted using the exact same Assessment Record. The new Assessment Program for both Suppliers and Materials will have the same format that previously had been associated with only a Material Assessment Program.
The new Assessment stages are highlighted in red and explained in detail below.
- Details
- Data Collection
- Nonconformance & Corrective Action
- Risk Assessment – Absence of Control
- Determination of Control
- Control Planning
- Risk Assessment – Presence of Control
- Approval
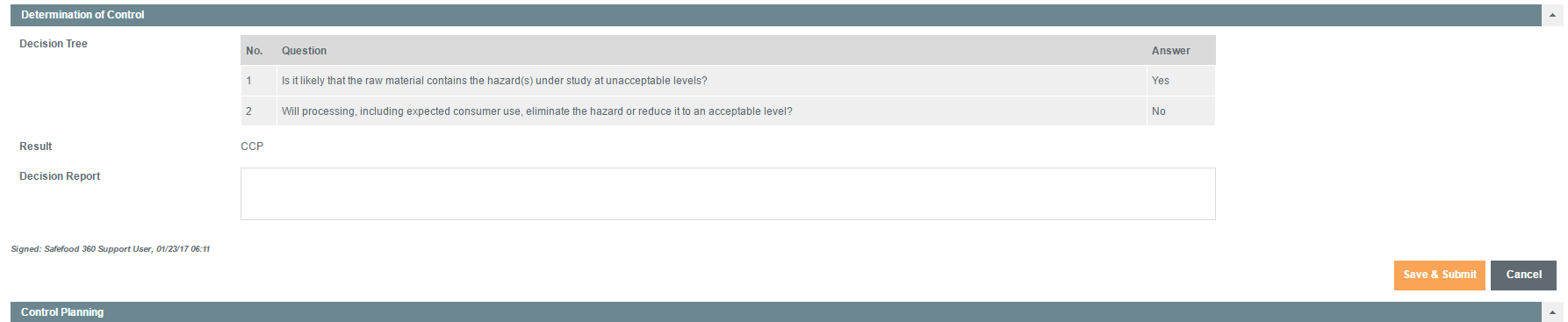
Determination of Control
Here, the appropriate controls which need to be applied are determined by using a structured decision tree.
Under FSMA, the requirement is clearly stated that, for significant hazards requiring a preventive control or a Supplier requiring a verification plan, a whole host of documents, data, and records must be obtained and reviewed on a frequently scheduled basis. This is the Data Collection stage in the workflow.
However, the legislation also provides for a whole host of conditions, exemptions, qualifications and other options, e.g., LACF, small producers, supplements, and recognized countries, etc.
This has created a very complex framework for the determination of controls which Safefood 360° users need to be aware of, as well as have clearly defined procedures to ensure the process of assessment accounts for them.
The adopted approach is based on the data collected by the Preventive Controls Qualified Individual (PCQI), following risk assessment, who will go through a series of questions in a pre-defined decision tree.
They will confirm, exactly, which sets of controls need to be applied to the Material and Supplier.
If there is a Critical Control Point (CCP), the program proceeds to the next stage, Control Planning, after ‘Save & Submit’ has been selected.
If it has been determined that there is not a CCP, the assessment will skip the next two stages and go to the final Approval stage.
Control Planning
This is where the specifics of the control measure(s) are detailed and defined for the Supplier or Material. Details that need to be defined are:
- Control name: A name which describes the activity e.g., Auditing.
- Control Limit: The specific point at which the product may become unsafe e.g, temperature, in the case of a heat treatment control.
- How: The means through which the control measure or procedure is conducted.
- Responsible: The person (or position) responsible for ensuring the control measure is conducted as per the specification and schedule.
- Frequency: How often the control measure needs to be conducted e.g., every two years in the case of audits.
- Corrective Action: The action to be taken in the event that the Critical Limits are not met.
- Records: The record where the data and evidence supporting the fulfillment of the control is maintained.
- Verification: The activity that confirms that all the elements of the control are in place, adequate, and capable of producing safe and legal food.
You can predefine Control Models in the software, and then insert these into your assessment by selecting the “Add Control” button. The Control Model is located in the Food Safety Plan Module > Add Model > Control Model.
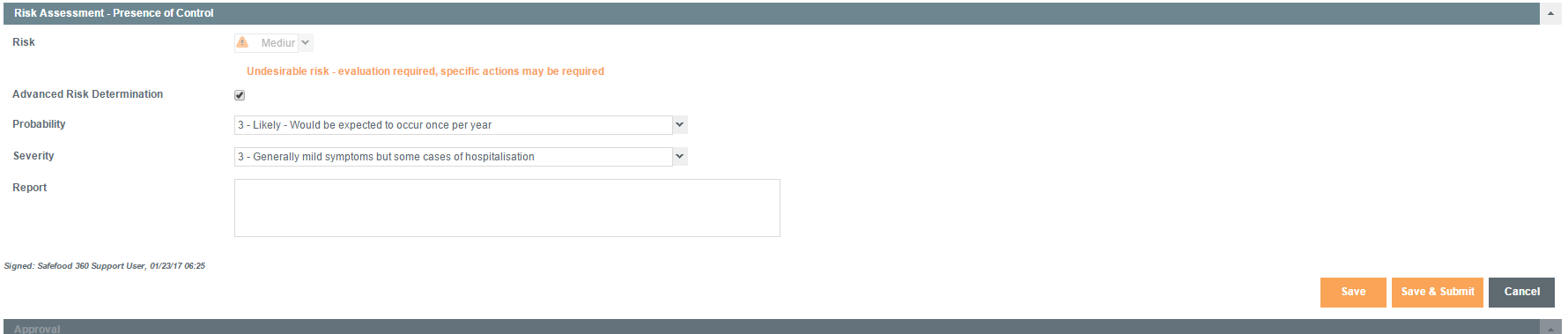
Risk Assessment – Presence of Control
In this phase, the PCQI makes a determination of the impact of the control measure(s) on the risk presented by the hazard.
In this case, the risk of the hazard should be reduced to an acceptable level, e.g., high to medium/medium to low.
Only Probability should change in this risk assessment since the control measures employed can only affect the Probability.
For example, in a heat treatment process designed to kill Salmonella, the heat treatment will reduce the Probability at which the pathogen will be present.
It does not, however, change the Severity of impact of Salmonella on the consumer, should it be present.
We are sure you will find these changes helpful on your path to becoming compliant with FSMA requirements. Feel free to let us know what you think in the comments below or through the software.



Leave a Reply
Want to join the discussion?Feel free to contribute!