Why FSMA is necessary
By now it should be no surprise to U.S. based food manufacturing companies that calling FSMA a comprehensive piece of legislation is putting it lightly.
We already had laws on the books, and HACCP-based food safety programs were widely used when GFSI hit our shores last decade the “food safety bar” was raised and companies were starting to get with the program.
In my previous blogs on food defense, and the foreign supplier verification program, as well as the examination of how HARPC differs from HACCP by George Howlett, the CEO of Safefood 360°, we pointed out that what previous legislation – and even GFSI schemes – lacked were the teeth necessary to compel companies to comply with the rules.
The failure to ensure that companies complied meant the level of safety and protection we deserved as consumers was lacking. It meant that something had to change.
The failure of food safety before FSMA
Theodore Roosevelt was credited with the phrase “speak softly and carry a big stick.” It could be argued that in the past, the FDA, despite its initial inception being founded under Roosevelt himself, spoke big, but food manufacturers came to understand that the stick it carried was disproportionately smaller.
The FDA spoke big but the stick it carried was disproportionately smaller.
That’s not to say that when the FDA came knocking on the door, companies wouldn’t sit up and make sure their best foot was forward while the inspector was on the premises, nor am I including all of the food companies under the same dirty blanket.
However, given the Senate committee hearings and high profile court cases we’ve all witnessed over the past 15 years, it’s apparent that enough companies felt the slackness in enforcement and decided to take unfortunate risks, which resulted in thousands of foodborne illnesses and even deaths!
This meant the market itself dictated the need for more stringent regulations and punitive enforcement.
A need for change
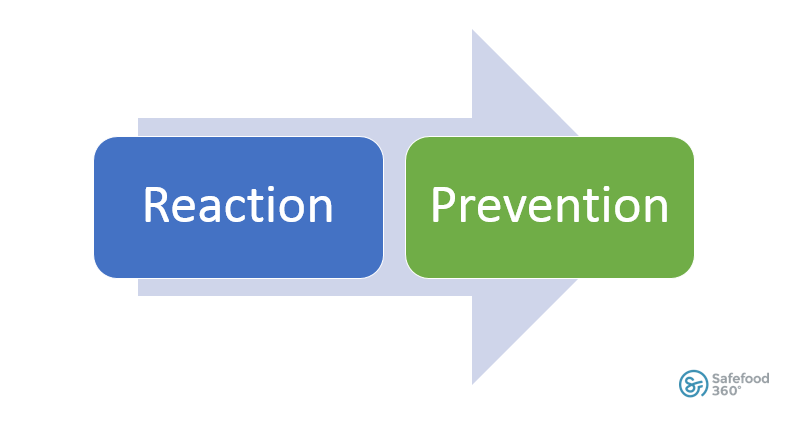
The FDA has distanced itself from the older method of simply reacting to issues as they arise.
FSMA requires food manufacturing companies to prevent issues from happening in the first place and, if a hazard arises, the responsibility is squarely on the shoulders of the food manufacturer.
The FDA has distanced itself from simply reacting to issues.
A number of years ago, I escorted an FDA inspector through a warehouse when he noticed more than one pallet of what appeared to be spoiled product.
In my attempts to downplay the matter, and reassure him that it was simply a low-level issue we were continuing to monitor, he insisted we take the next two weeks examining every container in the warehouse.
The FDA requested that we put all of the potentially affected product on hold pending the results of its investigation.
After a few weeks ̶ I’m being generous, it was more like a few months ̶ the results of the FDA investigation confirmed that it was a low-level anomaly, and we were to continue to monitor it just as we had been before their reactionary measures were introduced.
While I was able to breathe a huge sigh of relief at the time, and the prospects of remaining gainfully employed were back on track, this is a great example of how the FDA reacted to a situation instead of requiring that preventive controls be put in place which might have mitigated this low-level anomaly in the first place.
Whether it was an enhanced inspection, a form 483, or a warning letter, in many cases these proved to be little more than an inconvenience for the head of the QA Department. FSMA has given the FDA the tools to be more than an inconvenient bump in the road for a select number of individuals.
FSMA is more than an inconvenient bump in the road for a select number of individuals.
Giving the FDA teeth
FSMA strengthens the FDA in multiple ways. The new provisions granted under FSMA can be grouped into the following categories:
Preventive Measures
- Provides for the Expanded Administrative Detention of food
- The FDA has more authority to prevent adulterated or misbranded food from entering commerce.
- Increased Records Inspection
- Expanded access to records beyond those related to specific suspect articles of food believed to be adulterated or believed to present a threat of serious adverse health consequences, to records of any article of food that is reasonably suspected to be affected in a similar manner.
- Authority To Deny Entry
- Simply put, if a foreign food manufacturer / producer does not permit the FDA to inspect its facility, the FDA can refuse to allow any food from that facility to enter the U.S.
Enforcement Measures
- Suspension of Registration
- FSMA gives the FDA authorization to suspend a facility’s registration (under certain circumstances) if the food presents a serious health hazard.
- IMPORTANT: A facility with a suspended registration WILL NOT be able to legally sell any food until the FDA lifts the suspension! This applies for both interstate and intrastate.
- Mandatory Recall
- In the past, in the event of suspected contamination or adulteration, the FDA’s authority was limited to requesting the firm voluntarily recall the product. (Some might argue that a request to voluntarily recall your firm’s food carried a serious connotation; however, from a strict legal standpoint, it wasn’t overly forceful.) Under FSMA, the FDA can now ORDER a recall if the company does not cease distribution itself and recall the product.
Why FSMA is necessary
So, that answers the question regarding what the FDA’s new “teeth” are, but it doesn’t quite answer the question “Why is FSMA even required?” We can look at the CDC data regarding foodborne illness statistics to see that the nation’s food safety program and system of industry self-policing was broken. For most food safety managers, you’ve likely cited these statistics in your company’s annual training program.
As is human nature, when companies were given an inch of flexibility, they took a mile’s worth of liberty. There have been several high-profile legal battles because corporations put production goals ahead of food safety goals in that relaxed environment.
When companies were given an inch of flexibility, they took a mile’s worth of liberty.
Why do we need FSMA? It’s quite simple, really. We need FSMA because the consumers of the food produced in, or imported into, the United States are your grandparents, spouse, children, or grandchildren. If not yours, they are someone else’s grandparents, spouse, children, or grandchildren.
As food producers, the very least we can do is make every effort to ensure that the food they eat is safe.
I have the benefit of speaking to a wide range of companies across North America and I’m witness to companies at various stages of FSMA compliance readiness. I’m afraid that there are quite a few companies out there who have yet to understand that we are on the precipice of entering an era with a strengthened FDA, and the way food manufacturing has been done for the last century is changing.
Within the entirety of our food community, we had our failures.
So much so that our big brother has now stepped in to require us to be proactive instead of reactive.
For mine, and all of our future great-grandchildren’s sake, hopefully, this change is for the better!





Leave a Reply
Want to join the discussion?Feel free to contribute!