Novel Food Regulation:
EFSA vs. FDA
What defines Novel Food?
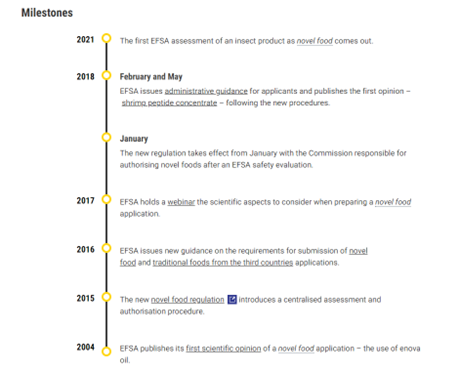
On February 10th, 2022, the European Commission authorized the placing on the market of a third insect, Acheta domesticus (house cricket), as a food. The term ‘house cricket’ refers to the adult of Acheta domesticus, an insect species that belongs to the Gryllidae family.
This authorization of the house cricket by the EFSA will allow the applicant to place this insect species on the EU market under certain conditions of use. This new Novel Food will consist of the frozen, dried, and powder forms of house cricket. It is intended to be marketed as a snack or as a food ingredient, in several food products.
How does the European Food Safety Authority classify Novel Foods?
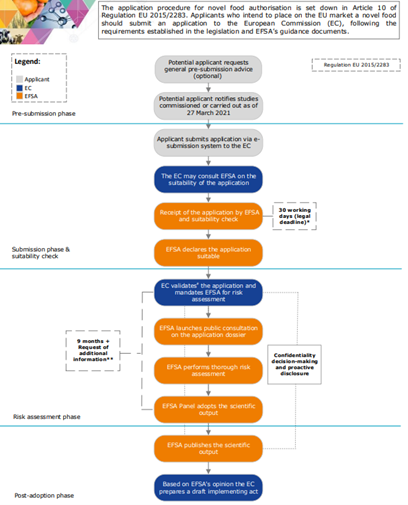
Since January 1st, 2018, the European Commission has been responsible for authorizing Novel Foods and, as part of the procedure, can ask the European Food Safety Authority (EFSA) to conduct a scientific risk assessment to establish their safety (EU Novel Food Regulation (EU) 2015/2283).
Pre-market authorization of Novel Foods based on an evaluation in line with the above principles is necessary.
In January 2021, the EFSA published its first completed assessment of a proposed insect-derived food product. The EFSA safety evaluations are a necessary step in the regulation of novel food, as their scientific advice supports EU and national decision-makers who authorize these products for the European market.
Below is a capture of the milestone since the first published scientific opinion by the EFSA on Novel Food application.
Placing Novel Foods on The Union List
Novel Foods should not be placed on the market or used in food for human consumption unless they are included in a Union list of Novel Foods authorized to be placed on the market within the Union (‘The Union List’).
According to the EU Regulation 2015/2283, criteria for the assessment of the safety risks arising from Novel Foods should be clearly defined and laid down. Under the procedure for authorizing a Novel Food and updating The Union List, the Authority should be requested to give its opinion if the update is liable to human health. The assessment includes also consideration of possible effects on vulnerable groups of the population. When a Novel Food is authorized and included in the Union List, the Commission should have the power to introduce post-market monitoring requirements to monitor the use of the authorized Novel Food to ensure that the use is within safe limits as established in the risk assessment by the Authority.
Article 4 of the above-mentioned regulation, set the procedure for the determination of the novel food status. The food business should:
- Verify whether the food which they intend to place on the market within the Union falls within the scope of this Regulation
- Where they are unsure whether a food that they intend to place on the market within the Union falls within the scope of this Regulation, food business operators shall consult the Member State where they first intend to place the novel food. Food business operators shall provide the necessary information to the Member State to enable it to determine whether food falls within the scope of this Regulation
An applicant who intends to place a novel food on the EU market should apply to the European Commission. After having verified its validity, the European Commission makes it available to the Member States and mandates the EFSA for a scientific assessment. The EFSA will issue an opinion within six months of a valid application receipt.
Below is a flow chart that represents the steps for the authorization of the novel food.
How are Novel Foods defined by the Food and Drug Administration?
The United States of America (USA) has different regulatory classification systems and pre-market approval processes. In the United States, no regulation defines “Novel Foods”; however, any new food ingredient is considered either as a food additive or Generally Recognized as Safe (GRAS).
A food additive is any substance that is reasonably expected to become a component of food either directly or indirectly; these require pre-market approval. In this case, the applicant needs to submit a Food Additive Petition (FAP) to the USA Food and Drug Administration (FDA). A food additive is any substance that is reasonably expected to become a component of food either directly or indirectly; these require pre-market approval. In this case, the applicant needs to submit a Food Additive Petition (FAP) to the US FDA.
GRAS substances, on the other hand, are exempted from the definition of “food additive” and instead are defined as “substances that are generally recognized, among experts qualified by scientific training and experience to evaluate their safety as having been adequately shown through scientific procedures to be safe under the conditions of their intended use.”
How does the FDA qualify a GRAS substance?
The Regulation states that any person may notify the FDA of a conclusion that a substance is GRAS under the conditions of its intended use.
A GRAS conclusion is based upon scientific procedures and requires the same quantity and quality of scientific evidence as is required to obtain approval for a food additive and direct the applicant to a series of guidance documents that address scientific issues associated with demonstrating the safety of a food substance. A GRAS conclusion based on common use in food requires evidence of a substantial history of consumption before January 1, 1958.
Furthermore, all GRAS conclusions must be considered in context based on the knowledge and information available at a point in time, because scientific knowledge and information about a particular substance can evolve and sometimes change over time. A food additive petition relies on privately generated data and information that are submitted to the FDA for review and determination of safety. The GRAS notification relies on broadly available, published information and data on the substance that can serve as the basis of the safety determination made by experts outside the FDA.
The notifier must address the safety of the notified substance, considering all animal food (including drinking water) as part of the animal’s total diet, considering any chemically or pharmacologically related substances in such a diet. In the explanation, the applicant must also address the safety of the notified substance regarding human exposure, considering all dietary sources and considering any chemically or pharmacologically related substances.
Furthermore, he must explain how the generally available data and information that he relies on to establish safety, provide a basis for the conclusion that the notified substance is generally recognized, among qualified experts, to be safe under the conditions of its intended use for both the target animal and for humans consuming human food derived from food-producing animals.
Some of the FDA scientific guidance documents are expressly directed to the evaluation of the safety of food additives. However, many of the recommendations in these guidelines could be useful to any person who evaluates whether a substance is GRAS under the conditions of its intended use. The FDA intends to re-visit these scientific guidance documents to determine whether and how to modify them to clarify that their guidance on evaluating the safety of a food substance applies regardless of whether the substance would be used in food as a food additive or as a GRAS substance.
The FDA’s response to a GRAS Notice has been in one of three categories:
- The agency does not question the basis for the notifier’s GRAS conclusion.
- The agency concludes that the notice does not provide a sufficient basis for a GRAS conclusion (e.g., because the notice does not include appropriate data and information or because the available data and information raise questions about the safety of the notified substance); or
- The response letter states that the agency has ceased to evaluate the GRAS notice at the notifier’s request.
In the GRAS’ final ruling, FDA noted the intention to maintain an Inventory of GRAS notices and the agency’s response to those notices, which continues the practice that began under a proposed rule published in 1997 (the GRAS proposal; 62 FR 19838). The GRAS Inventory page is the entry point to this information.
This inventory includes all filed GRAS notices since 1998, regardless of whether the notice is pending at FDA or has come to closure, and regardless of the nature of the FDA’s response. While the Regulatory framework is established for Novel Food and the evolution of food habits can offer new marketing frontiers for food business operators interested in novel food, the adaptation and therefore the consumption of Novel Food in the EU will probably occur more gradually. Certainly, later than in other member countries that do not have their own typical cuisine and are more open to innovation and experimentation in the food sector.
Food manufacturers need to be aware of food safety hazards in order to produce safe and high-quality food products. By downloading datasheets about food safety hazards, manufacturers can stay up-to-date on the latest information and make informed decisions about the food they produce. This helps to ensure the safety of their products and gives consumers peace of mind when purchasing and consuming food products.
Safefood 360° is an innovative software designed by food safety experts, and we are dedicated to providing solutions for a safer world for food manufacturers. Subscribe to our newsletter for the latest food safety news. If you would like more details about how to ensure the food safety of your company, submit a demo request here.






Leave a Reply
Want to join the discussion?Feel free to contribute!